Resources
The resources below can provide further support for healthcare professionals and patients living with pyruvate kinase (PK) deficiency regarding myAgios® Patient Support Services, treatments, and financial support:
Resources for Healthcare Professionals

PYRUKYND® Start Form
Enroll your patients in myAgios.

Annotated PYRUKYND Start Form
An overview of the myAgios PYRUKYND Start Form.
myAgios HCP Brochure
This brochure outlines all the education and support services provided by myAgios.
PYRUKYND Healthcare Professional Website
Explore the PYRUKYND website to learn more about dosing, efficacy and safety, and how PYRUKYND may help your patients with PK deficiency.
Know PK Deficiency Website
Learn more about PK deficiency and how it affects your patients.
Resources for Your Patients
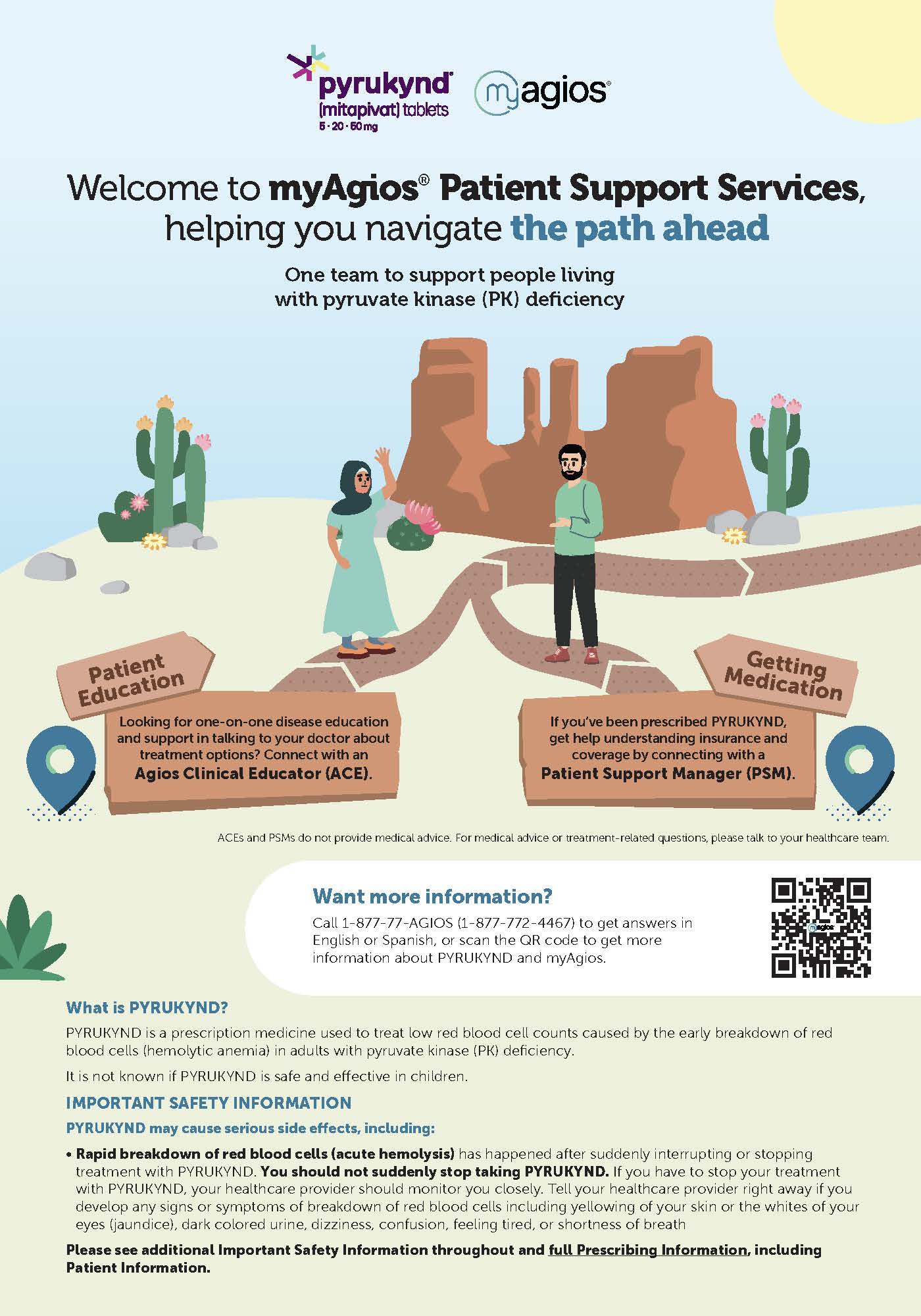
myAgios Patient Brochure
Helpful information for patients about PK deficiency, PYRUKYND, and myAgios.

PK Deficiency Patient Brochure
An overview of PYRUKYND treatment for PK deficiency and tools to help patients prepare for their next appointment.
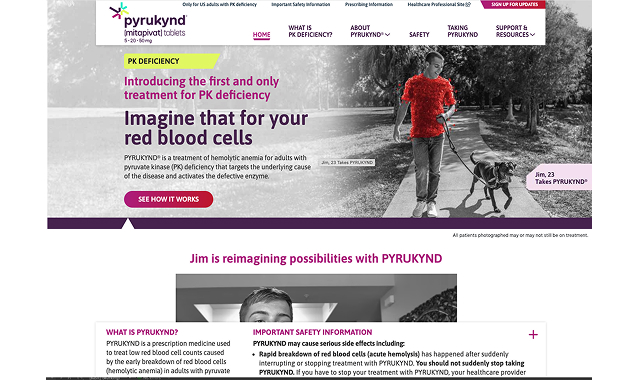
PYRUKYND Patient Website
Patients can visit the PYRUKYND website to learn all about how it works, how to take it, and how it may help them.
PK Deficiency Community

PYRUKYND patient stories
Hear from patients living with PK deficiency and learn about their experience with PYRUKYND.
PYRUKYND events
Join us for an in-person or online event to learn about PK deficiency and connect with people like you.